11. Potential allergens of green gram (Vigna radiata L. Millsp) identified as member of cupin superfamily and seed albumin |
No systematic study on allergenicity of green gram seed proteins has been performed so far, although incidences of IgE-mediated reaction to green gram seedlings have been reported. Authors sought to investigate the allergenic potential of green gram, followed by identification and characterization of its relevant allergens using proteomic approaches. BALB/c mice were sensitized intraperitoneally with green gram proteins, and levels of specific Igs, Th2 cytokines, histamine, anaphylactic symptoms and histopathological responses were studied. Twelve naso-bronchial allergic patients with a history of sensitization to green gram were selected on the basis of positive skin prick test and elevated specific IgE levels. Green gram allergens were identified and characterized by their ability to endure pepsin, by IgE immunoblot of two-dimensional (2D) gels in combina-tion with mass spectrometry and by bioinformatics approaches. Increased specific IgE, IgG1, Th2 cytokine and histamine levels, high anaphylactic scores and histological changes in lungs and spleen of green gram crude protein extract treated mice are indicative of its sensitization ability. Four proteins (molecular weights: 52, 50, 30 and 18 kDa) showed pepsin resistance and IgE-binding capability with sensitized human and mice sera. The four proteins tentatively named as Vig r2 (52 kDa, pI 5.7), Vig r3 (50 kDa, pI 5.8), Vig r4 (30 kDa, pI 6.6) and Vig r5 (18 kDa, pI 5.5) showed significant sequence similarity with known allergens of soybean, lentil, pea, lupin, etc. Mass spectrometric analysis identified Vig r2 as 8S globulin b-isoform precursor, Vig r3 as 8S globulin a-isoform precursor and Vig r4 as seed albumin. Green gram seeds contain at least four clinically relevant allergenic proteins, namely Vig r2, Vig r3, Vig r4 and Vig r5 that were capable of inducing strong IgE-mediated reactions. One of the most important steps towards diagnostic and therapeutic approaches to deal effectively with food allergy is continued identification of newer food allergens and their characterization. The significance of this study can be enormous as the data generated may work as basic biology data in developing a green gram species modified genetically that may have reduced allergenicity.
Misra et al.; Clinical & Experimental Allergy; 2011; 41;1157-1168. (Cited on Cover page)
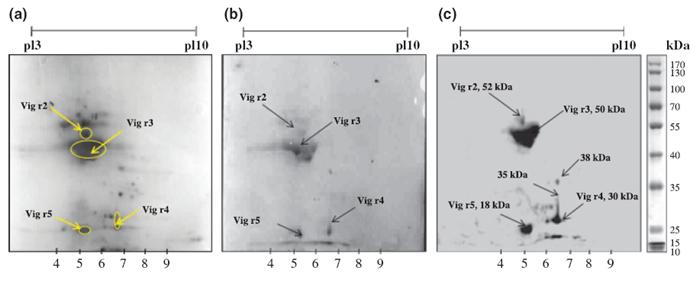 Two-dimensional (2D) electrophoresis of green gram crude protein extract (CPE) and IgE immunoblotting of 2D resolved proteins. (a) 2D SDSPAGE profile of green gram CPE. Green gram CPE were separated by isoelectric focusing (IEF) (pH 3–10) followed by SDS-PAGE (12%) and stained thereafter by CBB. (b) IgE-binding proteins detected by immunoblotting of 2D-resolved green gram CPE with pooled sera (1 : 20) of green gramsensitized mice. Horseradish peroxidase (HRP)-labelled secondary antibody (1 : 1000) directed to mouse IgE was used to visualize spots by enhanced chemiluminescence (ECL). (c) IgE-binding proteins detected by immunoblotting of 2D-resolved green gram CPE pooled serum of patients allergic to green gram. Sera from green gram-sensitive patients (1 : 20) were used as primary antibody; anti-human IgE-HRP (1 : 1000) was used to visualize spots by ECL. The encircled proteins corresponding to IgE-binding immunoblots were excised from 2D electrophoresed gel for mass spectrometry. |